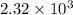Ideal gas law :
PV= nRT
where,
P = pressure
V= volume
n= number of moles
R= universal gas constant
T = temperature
Now, number of moles =
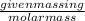
Put the value of number of moles in ideal gas law,
PV=
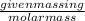 RT
RT
PV=
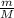 RT
RT
PM=
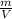 RT
RT
Density is the ratio of mass to the volume, thus, above equation is also written as:
PM=
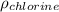 RT
RT
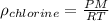
Put the values,
Temperature =
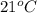 + 273 =
+ 273 =
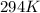
Pressure = 0.8 atm
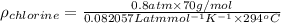
=
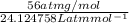
=
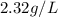
Now, specific gravity =
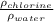
=
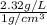
1 L = 1000
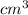
So, specific gravity =
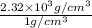
=
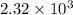
Thus, specific gravity is