For a general reaction,
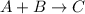
General expression for rate law will be:
![r=k[A]^(a)[B]^(b)](https://img.qammunity.org/2019/formulas/chemistry/college/xknetdlm670183e4cms4pyw3pgcy17l74n.png)
Here, r is rate of the reaction, k is rate constant, a is order with respect to reactant A and b is order with respect to reactant B.
The reaction is first order with respect to
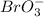 , second order with respect to
, second order with respect to
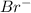 and zero order with respect to
and zero order with respect to
 .
.
According to above information, expression for rate law will be:
![r=k[BrO_(3)^(-)]^(1)[Br^(-)]^(2)[H^(+)]^(0)](https://img.qammunity.org/2019/formulas/chemistry/college/seludoj4czg368dg59z1dgbwxbcisu6g3u.png)
Or,
![r=k[BrO_(3)^(-)][Br^(-)]^(2)](https://img.qammunity.org/2019/formulas/chemistry/college/2r80ml2tt7wsgdqqlh6gvz7gq9kqwoxq1m.png) ...... (1)
...... (1)
- When concentration of
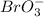 get doubled, rate of the reaction becomes,
get doubled, rate of the reaction becomes,
![r^(')=2k[BrO_(3)^(-)][Br^(-)]^(2)](https://img.qammunity.org/2019/formulas/chemistry/college/ml9xlf5nguuck4rek76ko63i9sq2tn1frp.png) ...... (2)
...... (2)
Dividing (2) by (1)
![(r^('))/(r)=(2k[BrO_(3)^(-)][Br^(-)]^(2))/(k[BrO_(3)^(-)][Br^(-)]^(2))=2](https://img.qammunity.org/2019/formulas/chemistry/college/tflawdavec66su4blvu6gskzt1eb5ocfdt.png)
Or,
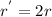
Thus, rate of the reaction also get doubled.
- When the concentration of
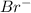 is halved, the rate of reaction becomes
is halved, the rate of reaction becomes
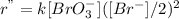
Or,
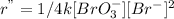 ...... (3)
...... (3)
Dividing (3) by (1)
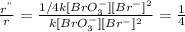
Or,
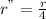
Thus, rate of reaction becomes 1/4th of the initial rate.
- When the concentration of
 is tripled:
is tripled:
Since, the rate expression does not have concentration of
 , it is independent of it. Thus, any change in the concentration will not affect the rate of reaction and rate of reaction remains the same as in equation (1).
, it is independent of it. Thus, any change in the concentration will not affect the rate of reaction and rate of reaction remains the same as in equation (1).