Step-by-step explanation:
A substance that is able to increase hydrogen ion concentration in water or a solvent is known as an acid.
For example, when HCl is dissolved in water then it will lead to release of hydrogen ions (
![[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/75vk4lm3dg3i0qv728qhnrrb5qib1loutc.png) ) or hydronium ions (
) or hydronium ions (
![[H_(3)O^(+)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/z87fpn7kestotksu3rnc4344ei1g9j0mtz.png) ) into water.
) into water.
The reaction will be as follows.
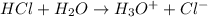
Thus, we can conclude that a substance that gives up a proton during a chemical reaction, raising the hydrogen ion concentration of water, is most appropriately called an acid.