The formula to determine the wavelength is, De-Broglie wavelength formula:
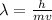 -(1)
-(1)
where,
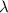 is wavelength, m is mass, v is velocity and h is Planck's constant =
is wavelength, m is mass, v is velocity and h is Planck's constant =
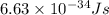 =
=
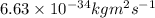
mass, m = 147 g (given)
Since, 1 g = 0.001 kg
So, 147 g = 0.147 kg
v = 91.0 mph (given)
Converting mph to mps:
Since,
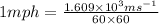
So,
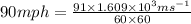 =
=
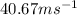
Substituting the values in formula 1:
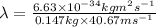
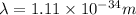
Hence, the wavelength wavelength of a 147-g baseball traveling at 91.0 mph is
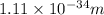 .
.