Given: Volume of flask=12 L
Number of moles of an ideal gas=1.3 mol
Temperature=20°C
Gas is an ideal gas thus, it follows the ideal gas equation as follows:
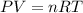
Here, P is pressure, V is volume, n is number of moles, R is Universal gas constant
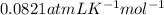 and T is temperature.
and T is temperature.
First convert temperature from °C to K as follows:
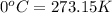
Thus,
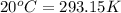
Rearrange ideal gas equation to calculate pressure,
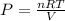
Putting the values,
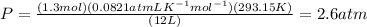
Therefore, pressure in the flask will be 2.6 atm.