Answer:
![r=-k[F_2][NO_2]](https://img.qammunity.org/2019/formulas/chemistry/college/7caj1hrhj7gkjtoz3q9ehk3zih1u2d99g3.png)
Step-by-step explanation:
Hello,
Based on the given information for the undergoing chemical reaction, its rate law is defined as:
![r=-k[F_2][NO_2]](https://img.qammunity.org/2019/formulas/chemistry/college/7caj1hrhj7gkjtoz3q9ehk3zih1u2d99g3.png)
This cloud be inferred due to the fact that on the statement, it is said that the reaction is first order in
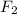 which means that its concentration is powered to the first power, in addition, since the overall order is two, the concentration of
which means that its concentration is powered to the first power, in addition, since the overall order is two, the concentration of
 is powered to first power as well, in order to the addition of each concentration's power turn out into 2 (two).
is powered to first power as well, in order to the addition of each concentration's power turn out into 2 (two).
Best regards.