A chlorine atom reacts with a sodium atom to form sodium chloride, NaCl. A chlorine atom can also react with another chlorine atom to form a chlorine molecule,
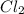
. Which statement BEST explains the behavior of chlorine in these two reactions?
A) A chlorine atom can form a covalent bond with another atom by sharing electrons.
B) A chlorine atom can form an ionic bond with another atom by accepting an electron.
C) A chlorine atom can form ionic bonds by accepting an electron and covalent bonds by sharing electrons.
D) A chlorine atom can form ionic bonds by sharing electrons and covalent bonds by accepting an electron.