Answer:- 3321 kJ of heat is released.
Solution:- The given balanced equation is:
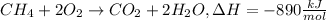
From this balanced combustion equation, 890 kJ of heat is released by the combustion of 1 mol of methane.
We could convert given grams of methane to moles and multiply by the delta H value of the reaction to get the total heat released by the combustion of 59.7 grams of methane as:
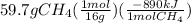
= -3321 kJ
The negative sign indicates the heat is released. So, 3321 kJ of heat is released by the combustion of 59.7 grams of methane.