Answer:- B. It's a dilute strong base.
Explanations:- The given concentration of calcium hydroxide is 0.02 M which is low means it is diluted. It has hydroxide ions which means it's a base. If it dissociates completely then it's a strong base. The dissociation equation is written as:
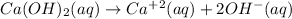
So, the correct option is B) It's a dilute strong base.