Answer:
D) FeO and CCl4 - One has charges that keep the ions bonded and the other does not.
Step-by-step explanation:
Hello,
In this case, for the iron (II) oxide, in order to know its formula, it is necessary to realize that the (II) indicates the oxidation state it has, it meas +2 from the roman number translation, in such a way, by knowing oxygen works with -2, therefore, the formula turns out:
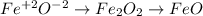
In addition, for carbon tetrachloride, the prefix tetra before chloride, points out there are four chlorined bonded to the carbon, that is why its formula is:
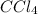
In such a way the answer is D) FeO and CCl4 - One has charges that keep the ions bonded and the other does not.
Best regards.