Answer:- 5.91%
Solution:- The given balanced equation is:
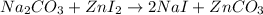
From given moles of zinc iodide we calculate the moles of zinc carbonate using mol ratio from the balanced equation and the moles are converted to grams on multiplying by molar mass.
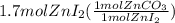
=
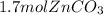
Molar mass of zinc carbonate is 125.38 gram per mol. Let's multiply the moles by molar mass to get the theoretical yield:
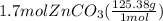
=
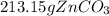
theoretical yield is 213.15 g and the actual yield is given as 12.6 g.
percent yield =
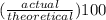
percent yield =
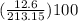
= 5.91%
The percent yield of zinc carbonate is 5.91%.