Answer:- C. 151 g
Solution:- The given balanced equation is:

From this equation, there is 1:1 mol ratio between all of them. We have been given with 117 grams of LIOH and 141 grams of HBr and asked to calculate the theoretical yield of LiBr.
let's convert the grams of each reactant to moles and see which one has limited moles as the theoretical yield is dependent on limiting reactant.
Molar mass of LiOH = 6.94 + 15.999 + 1.008 = 23.947 gram per mol
molar mass of HBr = 1.008 + 79.904 = 80.912 gram per mol
To get the moles of each reactant we divide it's grams by it's molar mass.
moles of LiOH =
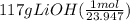 = 4.89 mol
= 4.89 mol
moles of HBr =
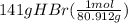 = 1.74 mol
= 1.74 mol
Moles of HBr are less means it is limiting reactant. There is 1:1 mol ratio between HBr and LiBr, so 1.74 moles of LiBr would form.
Molar mass of LiBr = 6.94 + 79.904 = 86.844 gram per mol
Mass of LiBr formed =
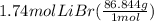 = 151 g LiBr
= 151 g LiBr
From calculations, theoretically 151 g of LiBr would form and so the correct choice is C.