Answer:- 147 mL.
Solution:- It is a dilution problem that could easily be solved using the dilution equation:
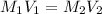
where,
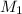 and
and
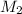 are the molarities of concentrated and diluted solutions and
are the molarities of concentrated and diluted solutions and
 and
and
 are their respective volumes. Let's plug in the given values in the equation and solve it for new volume that is
are their respective volumes. Let's plug in the given values in the equation and solve it for new volume that is
 .
.
Let's say the new volume is V. Let's plug in the values:
1.75M(84.0mL) = 1.00M(V)
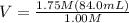
V = 147 mL
So, the volume of the diluted or new solution is 147 mL.