Answer: The correct answer is Xenon (Xe).
Step-by-step explanation:
Noble gases are defined as the gases which have fully filled electronic configuration or stable electronic configuration.
From the given options:
Option 1: Oxygen (O)
The atomic number of this element is 8.
Electronic configuration of this element:
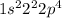
As, this is not the stable electronic configuration. Thus, it is not considered as a noble gas.
Option 2: Nitrogen (N)
The atomic number of this element is 7.
Electronic configuration of this element:
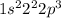
As, this is not the stable electronic configuration. Thus, it is not considered as a noble gas.
Option 3: Chlorine (Cl)
The atomic number of this element is 17.
Electronic configuration of this element:
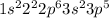
As, this is not the stable electronic configuration. Thus, it is not considered as a noble gas.
Option 4: Xenon (Xe)
The atomic number of this element is 54.
Electronic configuration of this element:
![[Kr]5s^24d^(10)5p^6](https://img.qammunity.org/2019/formulas/chemistry/college/q6yhkzvz82sgok9wmme5wbn0f6w5zcef6h.png)
As, this is a stable electronic configuration. Thus, it is considered as a noble gas.
Hence, the correct answer is Xenon (Xe).