Answer: The radius of vanadium atom is 132.07 pm
Step-by-step explanation:
To calculate the edge length of metal, we use the equation:
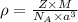
where,
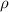 = density of metal =
= density of metal =
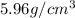
Z = number of atom in unit cell = 2 (BCC
M = atomic mass of metal = 50.9 g/mol
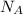 = Avogadro's number =
= Avogadro's number =

a = edge length of unit cell = ?
Putting values in above equation, we get:
![5.96g/cm^3=(2* 50.9)/(6.022* 10^(23)* (a)^3)\\\\a^3=2.836* 10^(-23)cm^3\\\\a=\sqrt[3]{2.836* 10^(-23)}=3.05* 10^(-8)cm^3=305pm](https://img.qammunity.org/2019/formulas/chemistry/college/c3eyo46obh4z5hbvaiyrwy1qym3z1e95eo.png)
To calculate the radius, we use the relation between the radius and edge length for BCC lattice:
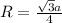
where,
R = radius of the lattice = ?
a = edge length = 305 pm
Putting values in above equation, we get:
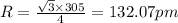
Hence, the radius of vanadium atom is 132.07 pm