Hydrochloric Acid (HCl) is a strong acid when it is present in the concentrated form but when it is dissolved in water (
 ) the atoms of this compound dissociates into its respective ions as shown below:
) the atoms of this compound dissociates into its respective ions as shown below:
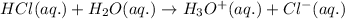
When we add HCl to any complex in its concentrated form the complex does not react at all but when its diluted to 6M and is kept for many hours, the complex reacts slowly. For eg:
![[Co(NH_3)_6]^3^+(aq.) + HCl(aq.) \rightarrow [Co(NH_3)_5Cl]^2^+(aq.) + NH_4^+ (aq.)](https://img.qammunity.org/2019/formulas/chemistry/college/dts49orf6gmnl5nw8k5gzsy5yd6ek61xr6.png)
As seen from the above reaction it can be seen the positive charge on the complex is reduced by 1 unit because one
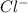 ion gets attached to the centre metal atom, therefore we can conclude that the charge on complex gets reduced by 1 unit when HCl reacts with the complex.
ion gets attached to the centre metal atom, therefore we can conclude that the charge on complex gets reduced by 1 unit when HCl reacts with the complex.