Answer: 6.6 moles of aluminium
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance weighs equal to its molecular mass and contains avogadro's number
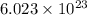 of particles.
of particles.
1 molecule of
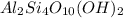 contains = 2 atoms of aluminium
contains = 2 atoms of aluminium
1 mole of
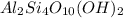 contains = 2 moles of aluminium
contains = 2 moles of aluminium
Thus 3.30 moles of
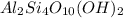 contain =
contain =
![\frac{2}{1]* 3.30=6.6]() moles of aluminium
moles of aluminium
Thus there are 6.6 moles of aluminium in the given compound.