Answer: Option (b) is the correct answer.
Step-by-step explanation:
A structural formula is defined as the formula that shows symbols of atoms present in the formula along with the bonds present between them.
For example, structural formula of ethene is
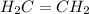 .
.
This structural formula shows that there exists a double bond between the two carbon atoms which the hydrogen atoms are attached to each carbon atom via single bond.
Whereas a chemical formula only represents the number of atoms present in a compound. But it does not tell about the arrangement of atoms.
For example, chemical formula of sulfuric acid is
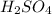 and this does not show how atoms are exactly arranged.
and this does not show how atoms are exactly arranged.
On the other hand, both space-filling model and ball-and-stick model tells us about how exactly atoms are arranged to each other. This is because both these models provide actual arrangement in a 3-dimensional structure of a compound or molecule.
Hence, with the help of space-filling model and ball-and-stick model it becomes easier to visualize the structure of a compound.
Thus, we can conclude that out of the given options structural formula provides the number and type of elements in a compound in the way that is close to their actual arrangement.