Formula units are another way of expressing "molecules". One mole is equivalent to
 .
.
Use dimensional analysis to solve this problem.
2.87 moles
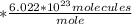
Then, cross off the moles in the given number and the denominator, and your remaining unit is molecules of sodium carbonate.
Then, multiply and divide accordingly to the fraction.
2.87 * 6.022*10^23 = 1.72 * 10^24. Notice how the given problem has only 3 sig figs, so the answer should also have 3 sig figs
Your final answer would be 1.72
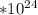 formula units of sodium carbonate.
formula units of sodium carbonate.