Mass of a cube of copper = 645 g
Density of copper = 8.96 g/cm3
Density of a substance is the mass of that substance per unit volume. i.e.
Density = Mass/Volume
Volume of the copper cube = Mass * Density
= 645 g * 8.96 g/cm3 = 5779.2 cm3
Now, if the length of the side of a cube is 'a' cm, then its volume (V) is :
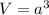
![a = \sqrt[3]{V}](https://img.qammunity.org/2019/formulas/chemistry/high-school/qi309yxij7wxivwbbuw3869om48gkirorf.png) =
=
![\sqrt[3]{5779.2}](https://img.qammunity.org/2019/formulas/chemistry/high-school/xvhqjqb1cpa2vqs914mimrxkultpw8wb2f.png) = 17.89 cm
= 17.89 cm