Answer:
The reaction is ionization.
Step-by-step explanation:
Ionization is a process in which molecules tends to lose or gain electrons thus acquiring positive or negative charge.
In the case of sodium chloride, acid and base reacts chemically to form the that compound. When hydrochloric acid
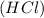 reacts with sodium hydroxide
reacts with sodium hydroxide
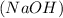 , the positive charged hydrogen
, the positive charged hydrogen
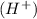 and negatively charged hydroxyl
and negatively charged hydroxyl
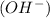 together form water and gets neutralized.
together form water and gets neutralized.
After evaporation of water, the positively charged sodium
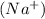 and negatively charged chlorine
and negatively charged chlorine
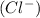 combines to form solid sodium chloride(NaCl).
combines to form solid sodium chloride(NaCl).