Answer: The correct answer is 'temperature will get doubled that is option D.
Step-by-step explanation:
Suppose the volume of the bubble is V, at temperature T and Pressure exerted by atmosphere P.
When bubble is blown with more air until the volume doubles the initial volume:
The new volume of the bubble = V'=2V
The new Temperature of the air inside the bubble will change to T'.
And the atmospheric Pressure remains same.
According Charles law:
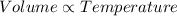 (At constant Pressure)
(At constant Pressure)
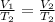
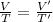
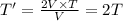
So, this means that temperature will get 2 times the initial temperature of the gas.
Hence, the correct answer is 'temperature will get doubled that is option D.