Answer: The atoms of carbon in the sample are
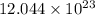 atoms.
atoms.
Step-by-step explanation:
To calculate the number of atoms, we will use mole concept.
According to the mole concept:
1 mole of an element contains
 number of atoms.
number of atoms.
We need to find the number of atoms of 2 moles of carbon, and it will be:
2 mole of carbon will contain
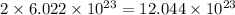 number of carbon atoms.
number of carbon atoms.
Hence, 2 moles of carbon contain
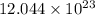 number of atoms.
number of atoms.