Answer : The correct option is, (A)
Explanation :
The given element is, fluorine is a non-metal with symbol (F). It is a gas and belongs to the group 17 and period 2.
The atomic number of fluorine = 9
The number of electrons = 9
The electronic configuration of fluorine will be,
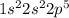
Hence, the correct option is, (A)