Answer:
Second option.
Step-by-step explanation:
Nitrogen is the first element of group 15, and its number atomic is 7, so, in its neutral state, it has 7 electrons.
By Linus Pauling diagram (see figure), the electronic distribution will be:
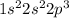
The distribution follows the arrows, from the less energetic subshell for the most energetic one. The subshell s has an only magnetic number, so only one orbital to put electrons. Subshell p has 3 magnetic numbers, so 3 orbitals to put electrons.
To put the electrons, we have to follow Hund's Rule: fulfill the orbitals which only one spin, until all orbitals have this spin, so continue, with the other spin, until finishing the electrons on that subshell. Spin is the rotation of the electron, and it can be +1/2 or -1/2.
So, for subshell 2p, how there are only 3 electrons, all three will be put with the same spin (+1/2, up arrow). If there was one more electron, it would be filled in the first orbital with the spin -1/2, down arrow.