Answer:- A) 3.17 mol.
Solution:- We have been given with 38.1 grams of C and asked to calculate the moles of carbon atom. For grams to moles calculations we divide the grams by the atomic mass of the element.
Atomic mass of carbon atom is 12.01 gram per mole of atom. Let's divide the given grams by the molar mass:
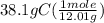
= 3.17 mole of C
So, the correct answer to this problem is choice A) 3.17 mol.