Answer :
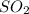 is the molecule that exhibit dipole-dipole forces as its strongest intermolecular force.
is the molecule that exhibit dipole-dipole forces as its strongest intermolecular force.
Explanation : The shape or geometry of given molecules of
 ,
,
 ,
,
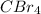 ,
,
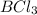 and
and
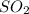 are linear, linear, tetrahedral, trigonal planar and angular respectively.
are linear, linear, tetrahedral, trigonal planar and angular respectively.
The given molecules
 ,
,
 ,
,
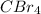 ,
,
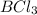 are symmetrical molecules. These symmetric molecules cannot exists as dipole even they contain polar bonds. Thus, these molecules will not exhibit dipole-dipole forces.
are symmetrical molecules. These symmetric molecules cannot exists as dipole even they contain polar bonds. Thus, these molecules will not exhibit dipole-dipole forces.
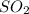 molecule has polar bonds and unsymmetrical bonds as shown in attached image. This molecule cannot exhibit H-bonding. It exhibit only dipole-dipole forces.
molecule has polar bonds and unsymmetrical bonds as shown in attached image. This molecule cannot exhibit H-bonding. It exhibit only dipole-dipole forces.
Therefore,
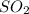 molecule exhibit dipole-dipole forces as strongest intermolecular force.
molecule exhibit dipole-dipole forces as strongest intermolecular force.