The balanced chemical equation for the reaction is as follows:
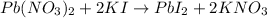
From the molarity and volume of
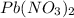 and KI, number of moles can be calculated as follows:
and KI, number of moles can be calculated as follows:
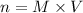
For
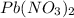 :
:
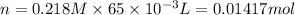
Similarly, for KI:
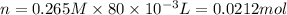
From the balanced chemical reaction, 1 mol of
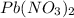 gives 1 mole of
gives 1 mole of
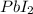 thus, 0.01417 mol will give 0.01417 mol.
thus, 0.01417 mol will give 0.01417 mol.
Molar mass of
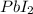 is 461.01 g/mol, mass can be calculated as:
is 461.01 g/mol, mass can be calculated as:
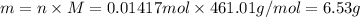
Similarly, 2 mol of KI gives 1 mole of
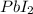 thus, 0.0212 mol will give
thus, 0.0212 mol will give
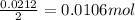 of
of
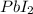 .
.
Mass can be calculated as:
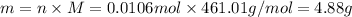
The amount of
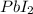 obtained from KI is less than that from
obtained from KI is less than that from
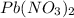 thus, KI is limiting reactant and amount of
thus, KI is limiting reactant and amount of
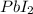 obtained from KI will be theoretical yield.
obtained from KI will be theoretical yield.
Actual yield is 3.26 g, % yield can be calculated as follows:
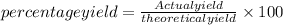
Putting the values,
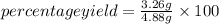 =66.80%
=66.80%
Therefore, % yield will be 66.80%.