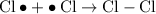A free-radical substitution reaction is likely to be responsible for the observations. The reaction mechanism of a reaction like this can be grouped into three phases:
- Initiation; the "light" on the mixture deliver sufficient amount of energy such that the halogen molecules undergo homologous fission. It typically takes ultraviolet radiation to initiate fissions of the bonds.
- Propagation; free radicals react with molecules to produce new free radicals and molecules.
- Termination; two free radicals combine and form covalent bonds to produce stable molecules. Note that it is possible for two carbon-containing free-radicals to combine, leading to the production of trace amounts of long carbon chains in the product.
Initiation
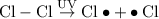
where the big black dot indicates unpaired electrons attached to the atom.
Propagation
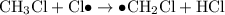
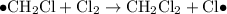
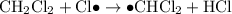
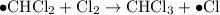
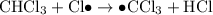
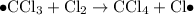
Termination