The molarity of formic acid is 100 mM or
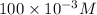 . The dissociation reaction of formic acid is as follows:
. The dissociation reaction of formic acid is as follows:
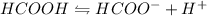
The expression for dissociation constant of the reaction will be:
![K_(a)=([HCOO^(-)][H^(+)])/([HCOOH])](https://img.qammunity.org/2019/formulas/chemistry/college/yqam3p70vmdrb6nimm4wosw9u7ao45awmj.png)
Rearranging,
![[HCOO^(-)]=(K_(a)[HCOOH])/([H^(+)])](https://img.qammunity.org/2019/formulas/chemistry/college/k8wzx07ru2t9a1q4r09kypo3ymubrk553p.png)
Here, pH of solution is 4.15 thus, concentration of hydrogen ion will be:
![[H^(+)]=10^(-pH)=10^(-4.15)=7.08* 10^(-5)M](https://img.qammunity.org/2019/formulas/chemistry/college/jc9lorl5oyehr9hxl1rqtwsxkwssb4p5wo.png)
Similarly,
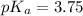 thus,
thus,
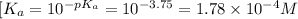
Putting the values,
![[HCOO^(-)]=((1.78* 10^(-4)M)(100* 10^(-3)M))/((7.08* 10^(-5)M)=0.2511 M](https://img.qammunity.org/2019/formulas/chemistry/college/a4ga6cx1b681sn0aolss9wt93iroiphc8j.png)
Therefore, the concentration of formate will be 0.2511 M.