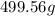The mathematical expression for depression in freezing point is given as:
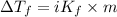
where,
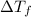 = depression in freezing point
= depression in freezing point
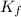 = molal depression constant (
= molal depression constant (
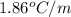 )
)
m = molality
i = Van't Hoff factor
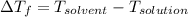
=
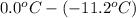
=
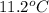
Now, put the values in formula,
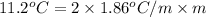 (as sodium chloride dissociate into two ions, i =2)
(as sodium chloride dissociate into two ions, i =2)
m =
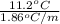
=
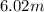
Now, molality of the solution =
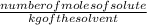
Number of moles =
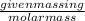
Molar mass of sodium chloride = 58.44 g/mol
molality of the solution =
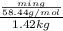 (1 L = 1kg)
(1 L = 1kg)
6.02 m =
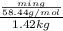
mass in g =
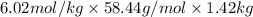
=
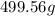
Thus, mass of sodium chloride is