Answer:- 14.0 moles of hydrogen present in 2.00 moles of
![[tex]C_6H_7N](https://img.qammunity.org/2019/formulas/chemistry/middle-school/bqd9wj9ptqgj9j9fhhpx6j2t069orobp9b.png) .
.
Solution:- We have been given with 2.00 moles of
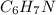 and asked to calculate the grams of hydrogen present in it. It's a two step conversion problem. In first step we convert the moles of the compound to moles of hydrogen as one mol of the compound contains 7 moles of hydrogen. In next step the moles are converted to grams on multiplying the moles by atomic mass of H. The calculations are shown as:
and asked to calculate the grams of hydrogen present in it. It's a two step conversion problem. In first step we convert the moles of the compound to moles of hydrogen as one mol of the compound contains 7 moles of hydrogen. In next step the moles are converted to grams on multiplying the moles by atomic mass of H. The calculations are shown as:

= 14.0 g H
So, there are 14.0 g of hydrogen in 2.00 moles of
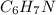 .
.