Eutectic point indicates the lowest possible melting temperature of the compound formed from mixture of compounds.
According to Eutectic point the resulting melting temperature of mixture is lower than the specific compound mixed.
Melting temperature of phenol is 40.5 C whereas for diphenylamine is 53 C The mean of melting temperature of mixture would be
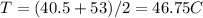
First thing the resulting temperature supposed to be lower than both temperature of phenol and diphenylamine means the resulting temperature should be maximum reach upto 40.5 C.
There is a difference of 12.5 C between the melting temperature of compounds, so the lowest possible temperature of mixture would be (40.5 – 12.5 = 28 C)
As, 85 Mol % phenol is present in mixture so the resulting temperature of mixture should follow the pattern of temperature of phenol. As, the melting temperature of phenol is lower than diphenylamine and thus the melting temperature of mixture would be the lower temperature 28 C otherwise it gets to higher temperature of 40.5 C.
Thus, the possible range of mixture would be near about 28 C so to make it between 26 – 30 C.