Answer: Option (C) is the correct answer.
Step-by-step explanation:
Whenever heat is lost in a chemical reaction or by a substance then the value of
 will be negative.
will be negative.
And, when heat is absorbed in a chemical reaction or by a substance then the value of
 will be positive.
will be positive.
As change in temperature is calculated as follows.
 = Final temperature - Initial temperature
= Final temperature - Initial temperature
So, when the value of initial temperature is raised and value of final temperature is lowered then the final change in temperature will come out to be negative.
This will show that heat lost by the piece would be higher.
Therefore, we can conclude that heat lost by the piece would be higher if the initial temperature is raised and the final temperature is lowered by
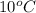 .
.