We are given volume of gasoline = 15.58 L and
Density = 0.74 g/mL.
Density is given in grams(g) per milliliter (mL). So, we need to convert given volume in mL also.
We know, 1 litre = 1000 milliliters
Therefore, 15.58 L = 15.58 * 1000 mL = 15580 mL
We could rewrite,
Volume of gasoline = 15580 mL.
Formula for density is..
Density (ρ)=
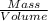
Plugging values in formula,
0.74 g/mL =
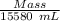
Muliplying both sides by 15580.
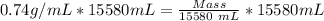
11529.2g = mass.
Therefore, 11529.2g grams does 15.58 L of gasoline weigh.