Step-by-step explanation:
According to Henderson-Hasselbalch equation:
pH =
![pK_(a) + log(([TRIS])/([TRISH^(+)]))](https://img.qammunity.org/2019/formulas/chemistry/college/as031cmqfdykxoxukq8h6w28lr7udclfzj.png)
When pH = pKa = 8.
Therefore,
8 = 8 +
![log(([TRIS])/([TRISH^(+)]))](https://img.qammunity.org/2019/formulas/chemistry/college/wmaunyy8y9hurqvx4res5h51gnyc70xt63.png)
![log(([TRIS])/([TRISH^(+)]))](https://img.qammunity.org/2019/formulas/chemistry/college/wmaunyy8y9hurqvx4res5h51gnyc70xt63.png) = 0
= 0
![([TRIS])/([TRISH^(+)])](https://img.qammunity.org/2019/formulas/chemistry/college/kyju6rp80ffcj8pu82bwb5pl3ic2j6whsd.png) =
=
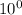
= 1
Hence, [TRIS] =
![[TRISH^+]](https://img.qammunity.org/2019/formulas/chemistry/college/6w5bvd2qrc4ocy51rpvg2p2xm78cqhcaal.png)
As, fraction of amine groups converted to
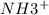
=
![([TRISH^+])/(([TRISH+] + [TRIS]))](https://img.qammunity.org/2019/formulas/chemistry/college/i5osvpmbnmggkbuzeyr5tcrfoa4wp125nj.png)
=
![([TRISH^+])/(([TRISH+] + [TRISH^+]))](https://img.qammunity.org/2019/formulas/chemistry/college/dfwfsrtbijex3e5i49t63hv8tivo42eiib.png)
=
![([TRISH^+])/((2[TRISH^+]))](https://img.qammunity.org/2019/formulas/chemistry/college/97izfmcnt4agkngkt2m29xpf1l4duw5zgq.png)
=
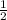
= 0.5
Hence, we can conclude that fraction of the amine group coverted to
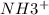 is 0.5.
is 0.5.