Answer: 52.00 amu.
Explanation:
Mass of isotope 1 = 49.94605 amu
% abundance of isotope 1 = 4.350% =
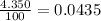
Mass of isotope 2 = 51.94051 amu
% abundance of isotope 2 = 83.79 % =
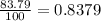
Mass of isotope 3 = 52.94065 amu
% abundance of isotope 3 = 9.500 % =
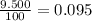
Mass of isotope 4 = 53.93888 amu
% abundance of isotope 4 = 2.360 % =
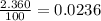
Formula used for average atomic mass of an element :
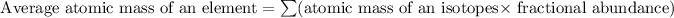
![A=\sum[(49.94605* 0.0435)+(51.94051* 0.8379)+(52.94065* 0.095)+(53.93888* 0.0236)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/jwzp8e5o1r3pojitd3nb5c11ts4dwhm3cb.png)
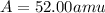
Therefore, the average atomic mass of an element is 52.00 amu.