Mass of potassium hydrogen pthalate KHP is 0.4856 g, its molar mass is 204.22 g/mol, number of moles of KHP can be calculated as follows:
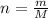
Here, m is mass and M is molar mass, putting the values,
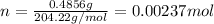
This will be number of moles of NaOH at equivalent point.
Detailed calculations:
Molarity is defined as number of moles in 1 L of solution, for 250 mL of solution, molarity will be:
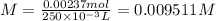
For 25 mL, apply dilution law as follows:
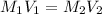
Putting the values,
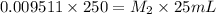
On rearranging,
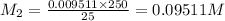
Convert molarity into number of moles,
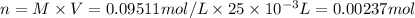
At equivalent point, number of moles of KHP will be equal to NaOH, thus, number of moles of NaOH will be 0.00237 mol.
Calculation for molarity:
Volume of NaOH is 18.75 mL, thus, molarity can be calculated as follows:
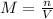
Putting the values,
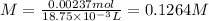
Therefore, molarity of NaOH is 0.1264 M