Heat is equal to product of mass, change in temperature and specific heat.
The formula is given by:
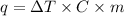
where,
q = heat
 = change in temperature that is
= change in temperature that is
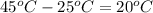
m = mass of substance
C= specific heat capacity
Put the values,
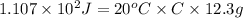
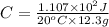
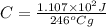
=
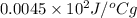
=
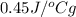
Specific heat capacity is equal to
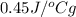 and this value corresponds to element iron.
and this value corresponds to element iron.
Thus, the unknown substance is iron.