Empirical formula of a compound gives the proportions of the elements in that compound but it does not define the actual arrangement and number of atoms.
Let the empirical formula of compound be
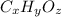 .
.
The ratio of number of moles of C, H and O can be calculated as follows:
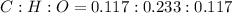
Simplifying the ratio,
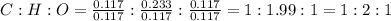
Thus, the value of x, y and z will be 1, 2 and 1 respectively.
Therefore, the empirical formula will be
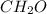 .
.