If it is saturated compound then we can calculate the double bond equivalent which will be equal to the number of rings in the compound
the double bond equivalent can be calculated using following formula
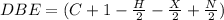
Where
H = number of Hydrogen atoms
C = number of carbon atoms
X= number of halogen atoms
N = number of nitrogen atoms
DBE = (10 + 1 - 16 / 2 ) = 3
Hence there are three rings in the compound