Answer:
Ethanol and dimethyl eher.
Step-by-step explanation:
Hello,
Based on the molecular formula,
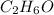 , two possible isomers could occur, ethanol and diethyl ether and are shown on the attached picture.
, two possible isomers could occur, ethanol and diethyl ether and are shown on the attached picture.
Ethanol is a saturated organic alcohol containing two carbons, six hydrogens and one oxygen; it is widely used in the alcoholic beverages industry and is obtained via ether synthetic or biochemically. On the other hand, dimethyl ether has the same types and numbers of atoms; it is widely used as an organic solvent employed in liquid-liquid extraction processes.
Best regards.