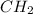The combustion reaction are as follows.
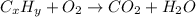
From the given:
Amount of carbon dioxide = 33.01 g
Amount of water = 9.02 g
Let's calculate the number of moles of each compound in the reaction
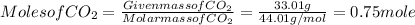
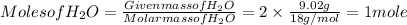
Each value is divided by small value
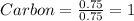
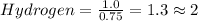
Therefore, Empirical formula of given hydrocarbon is