The formula of molarity is:
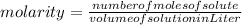
Number of moles is given as:
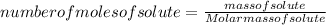
So, the formula of molarity can be written as:
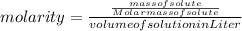 -(1)
-(1)
Molar mass of ethyl alcohol,
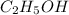 =
=
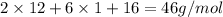
Molarity of ethyl alcohol,
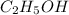 = 1 molar (given)
= 1 molar (given)
Let the volume of solvent that is water be 1 L.
Substituting the values in formula (1):
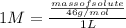
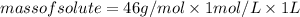
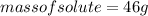
Since, ethyl alcohol is liquid in room temperature:
Density of ethyl alcohol is
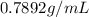
Density =
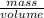
So, volume of ethyl alcohol =
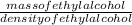
Volume of ethyl alcohol =
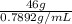
Volume of ethyl alcohol =
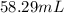
Hence, by dissolving 58.29 mL of ethyl alcohol in 1 L of water, 1 M solution of ethyl alcohol can be prepared.