The volume of water displace by crown is 10.7 mL. This will be equal to the volume of crown. Mass of crown is 112 g.
Density of a substance is defined as mass of substance per unit volume. It is mathematically represented as follows:
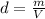
Putting the values,
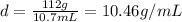
The theoretical value of density of silver is
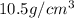 also, 1 mL is equal to
also, 1 mL is equal to
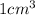 thus, density will be 10.5 g/mL.
thus, density will be 10.5 g/mL.
This is approximately equal to the calculated value thus, the crown is made up of silver.