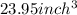Density of titanium is
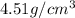 and mass given is 3.9 lb. First convert mass from pound to gram as follows:
and mass given is 3.9 lb. First convert mass from pound to gram as follows:
1 lb=453.592 g
Thus,
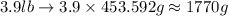
Density of a substance is defined as mass per unit volume. Thus, volume can be calculated as follows:
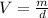
Putting the values,
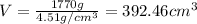
Converting the volume into cube inches:
Since, 1 cm=0.3937 inch
thus,
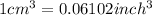
and,

Therefore, volume of titanium in cube inches will be