Answer: The atomic mass of gallium-71 is 70.92 amu.
Step-by-step explanation:
Mass of isotope Ga-69 = 68.9256 amu
% abundance of isotope Ga-69 = 60.11% =
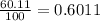
Mass of isotope Ga-71 = ?
% abundance of isotope Ga-71 = (100-60.11)% =
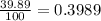
Formula used for average atomic mass of an element :
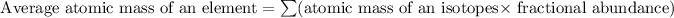
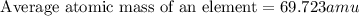
![69.723 =\sum[(68.9256* 0.6011)+(x* 0.3989)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/89foxhd20yyqi52suutc0bt9xpiy6xqj48.png)
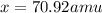
Therefore, the atomic mass of gallium-71 is 70.92 amu.