Step-by-step explanation:
A chemical reaction equation that contains same number of atoms on both reactant and product side is known as a balanced chemical equation.
For example,

Number of atoms present on reactant side are as follows.
N = 1
H = 3
O = 2
Number of atoms present on product side are as follows.
N = 1
H = 2
O = 2
Hence, in order to balance this equation we multiply
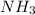 by 2 and
by 2 and
 by
by
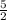 on reactant side. Whereas multiply NO by 2 and
on reactant side. Whereas multiply NO by 2 and
 by 3 on product side.
by 3 on product side.
Therefore, the balanced chemical reaction equation is as follows.
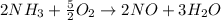
So, coefficients of reactants are 2 and
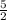 . On the other hand, coefficients of products are 2 and 3.
. On the other hand, coefficients of products are 2 and 3.