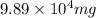First, calculate the mass of sodium in g with the help of molar mass and number of moles.
Number of moles =
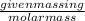 (1)
(1)
Molar mass of sodium =
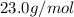
Substitute the given value of number of moles and molar mass of sodium in formula (1)
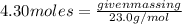 (1)
(1)
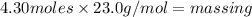 (1)
(1)
mass of sodium in g =
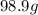
Now, according to conversion factor, 1 g = 1000 mg
So,
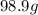 of sodium =
of sodium =
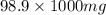
=
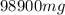 of sodium
of sodium
Thus, mass of sodium in mg =