Answer: Option (c) is the correct answer.
Step-by-step explanation:
A non-polar covalent bond is defined as the bond formed due to equal sharing of electrons between the combining atoms.
For example, in a
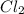 molecule there will be sharing of only one electron by each chlorine atom. This is because here electronegativity difference is zero so, there will be no attraction of electrons by either of the chlorine atoms.
molecule there will be sharing of only one electron by each chlorine atom. This is because here electronegativity difference is zero so, there will be no attraction of electrons by either of the chlorine atoms.
Hence, no charges will tend to develop.
A polar covalent bond is formed when there occurs unequal sharing of electrons between the two combining atoms.
For example, in HCl the chlorine is more electronegative in nature. Hence, it will attract the electron from hydrogen atom more towards itself. As a result, partial charges will develop.
An ionic bond is defined as the bond formed due to sharing of electrons between a metal and a non-metal.
For example, KCl is an ionic compound.
Thus, we can conclude that the type of bond produced when atoms share electrons equally is a non-polar covalent bond.